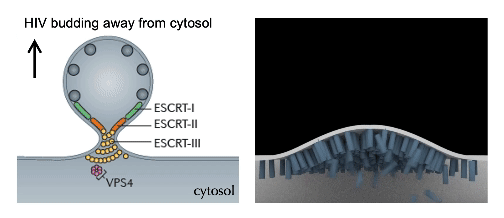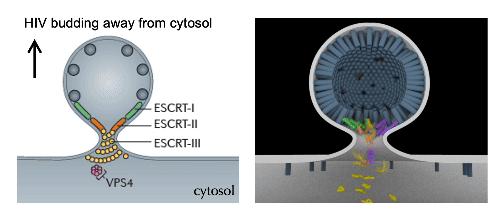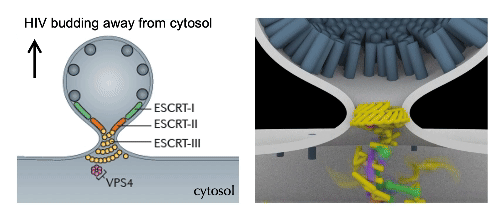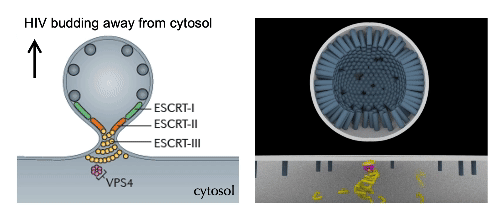
cellular mechanics and Force Dynamics
Cell-Bio-Physics
Cells and biomolecules (DNA, RNA & proteins) are physical objects possessing unique mechanical properties and responding to specific mechanical cues such as tensions and forces. Their physical characteristics critically impact how they carry out basic operations in biological processes over a broad range of scales, e.g., from chromosome segregation during mitosis, nuclear mechano-transduction in cell lineage differentiation, to embryo development adapting shape and morphology. Yet, this mechanical dimension in biology has often been overlooked — partly due to limited experimental approaches available — and holds great potential to resolve aberrant phenomena unrelated to genomic mutations as found in certain cancer cell proliferation and tumorigenesis.
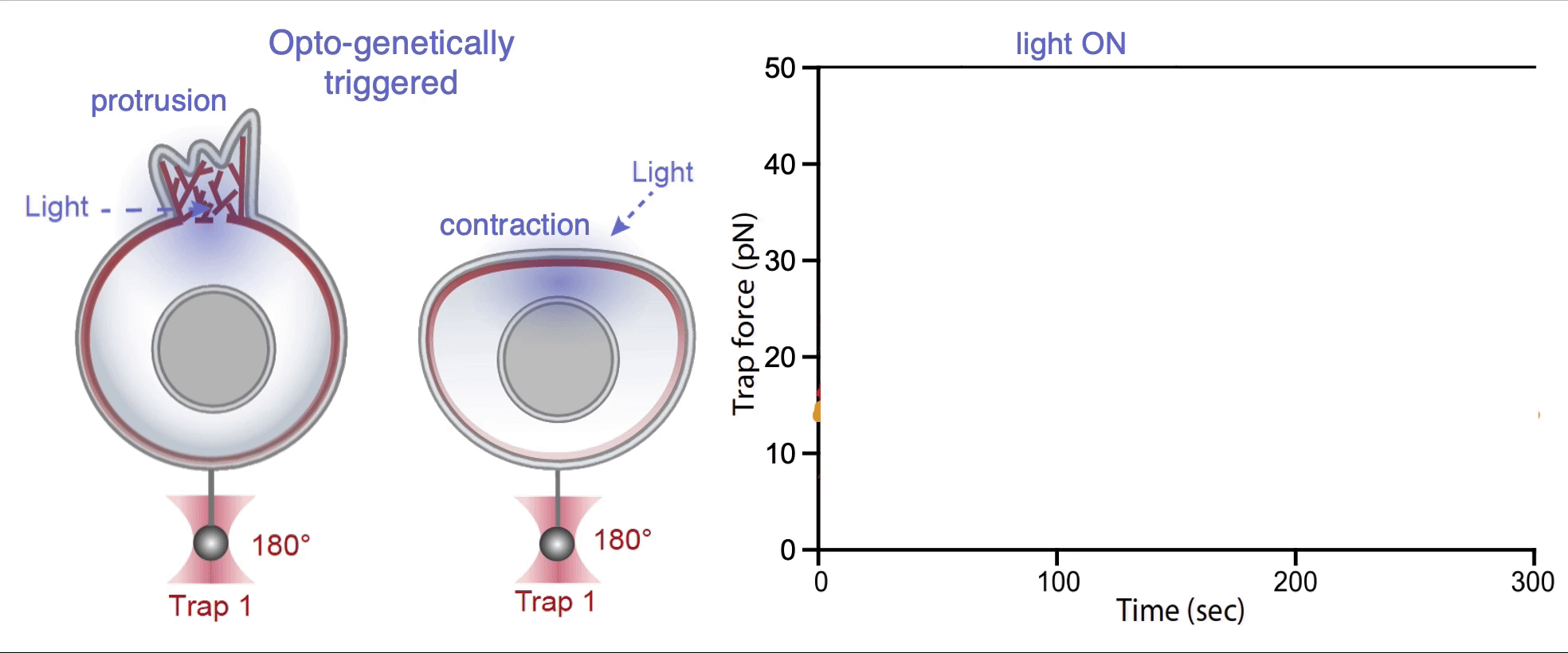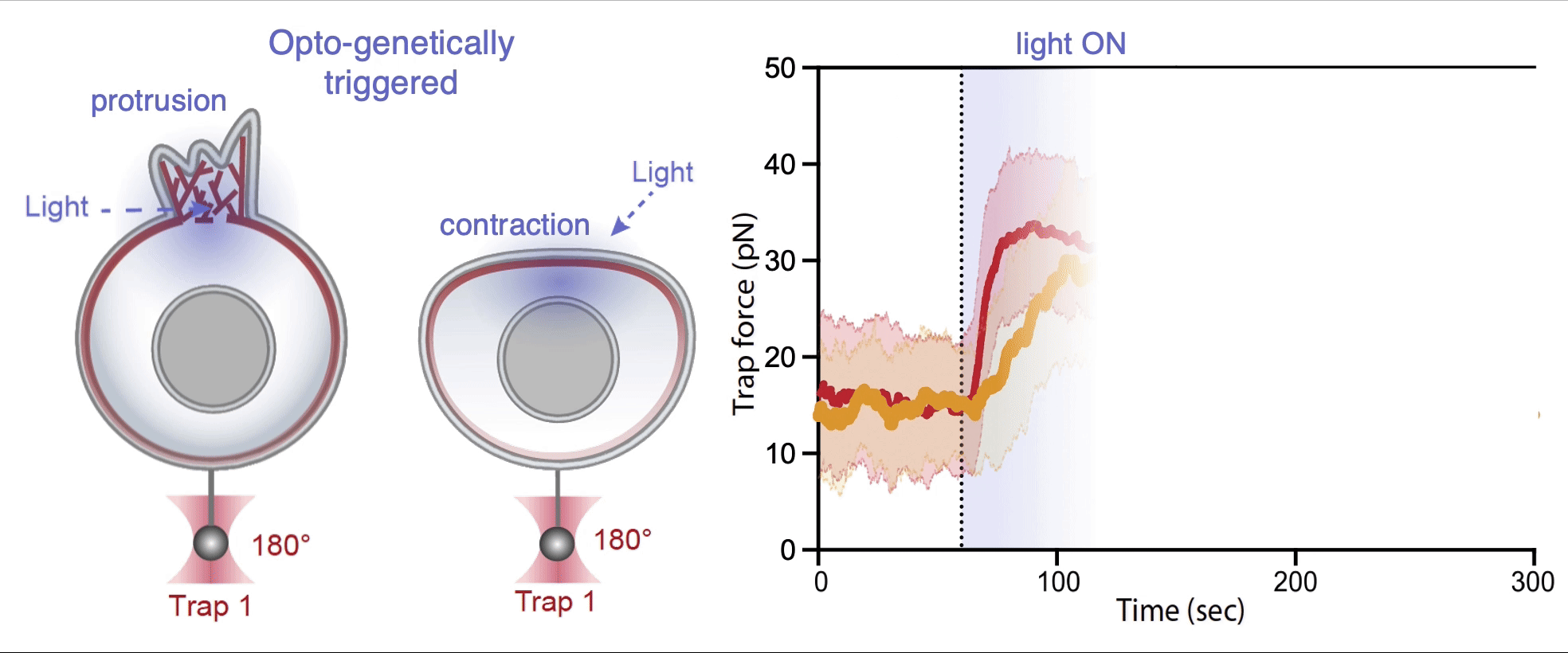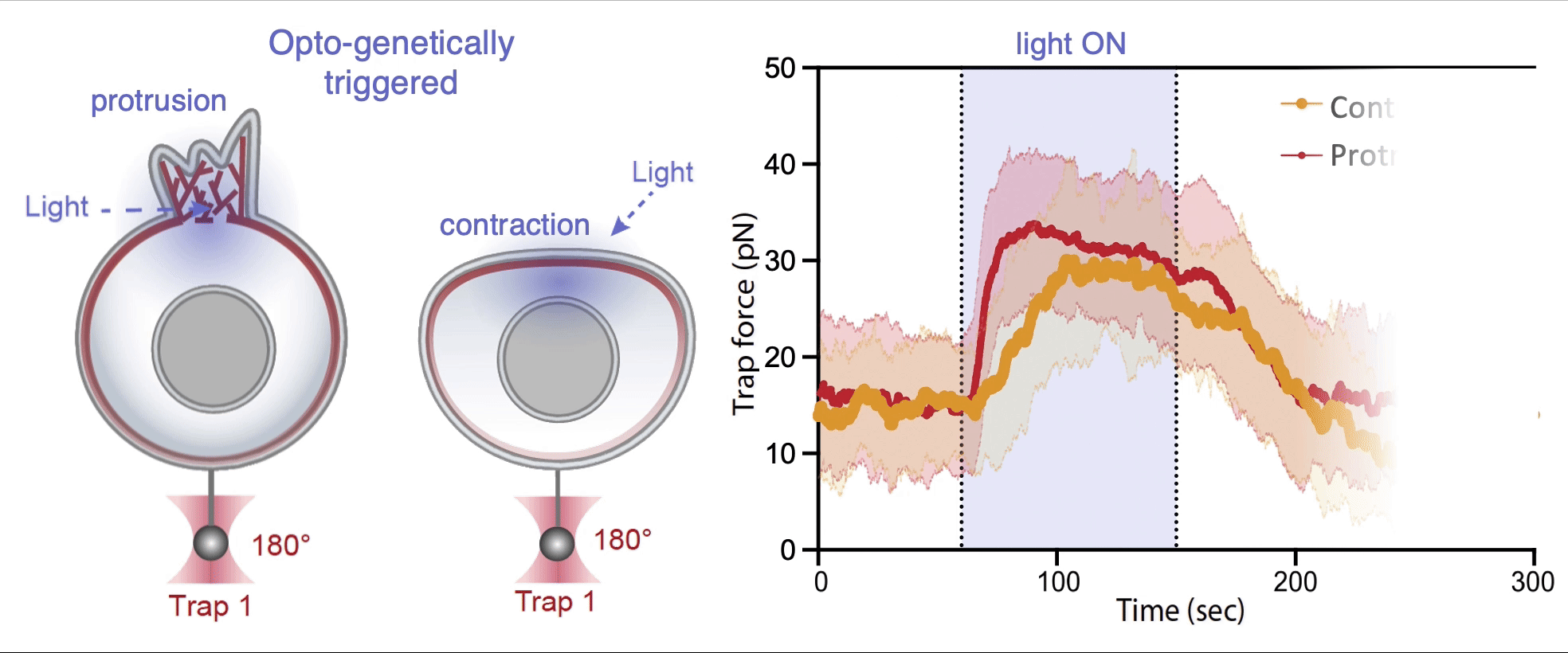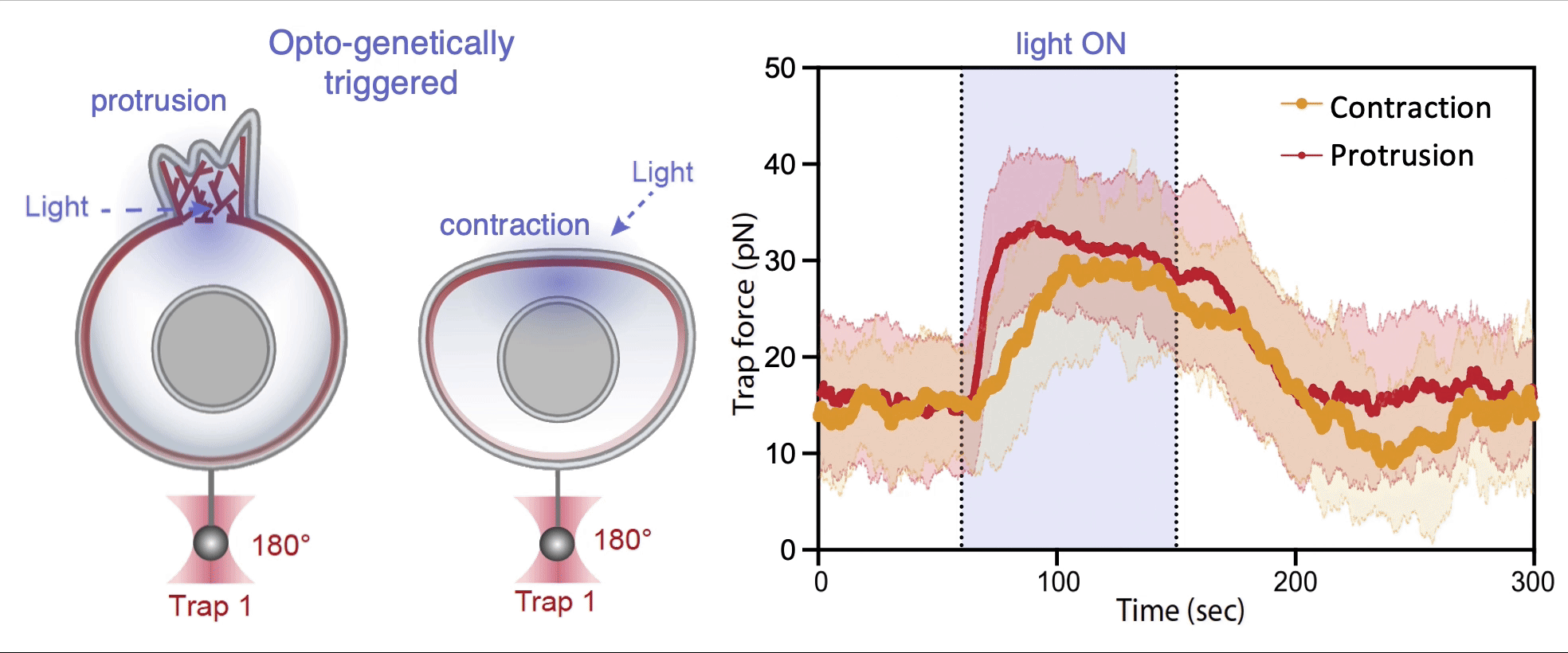
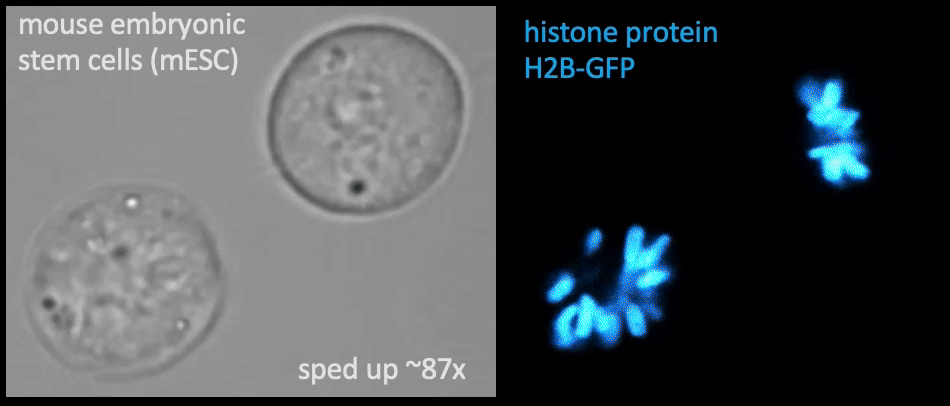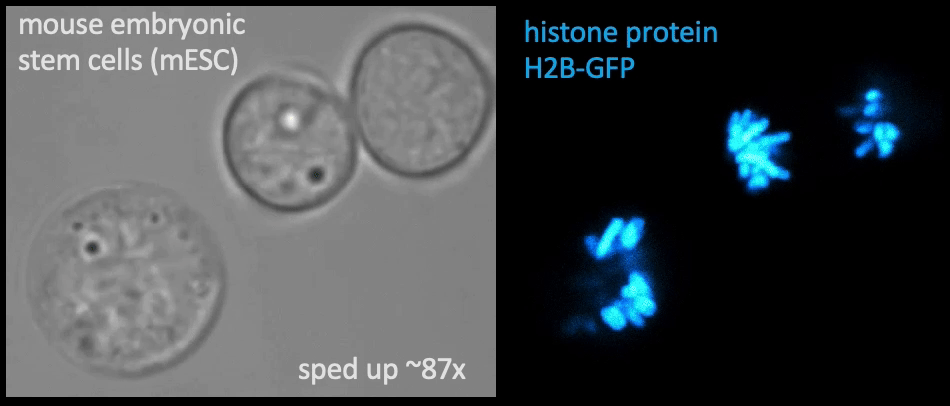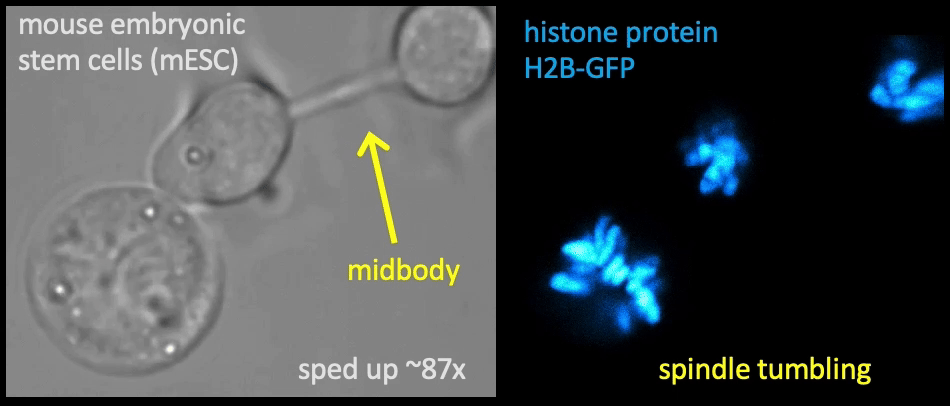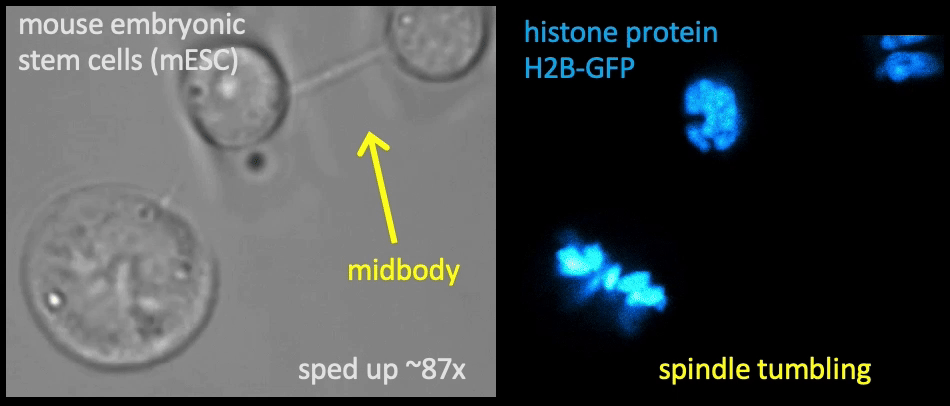
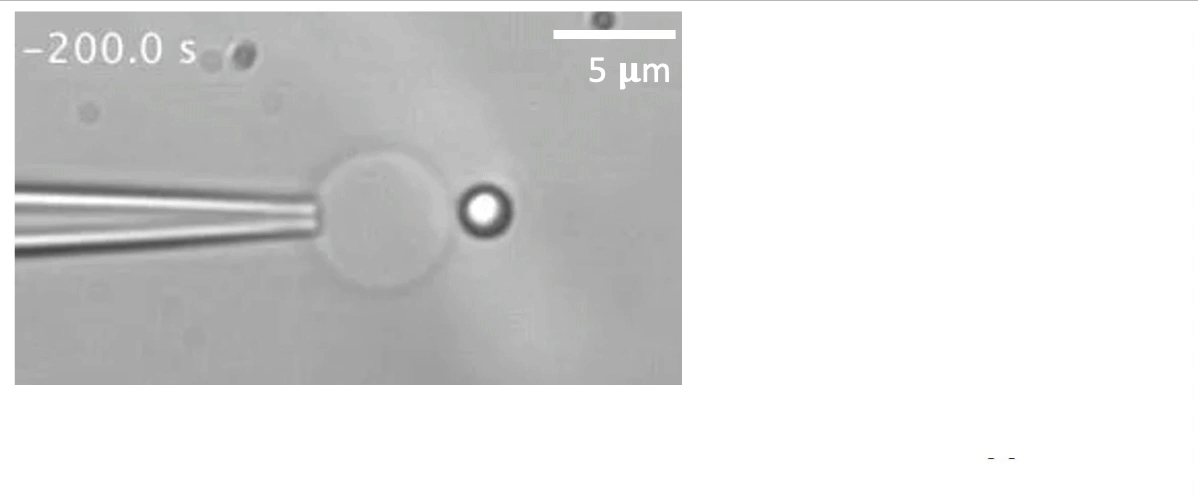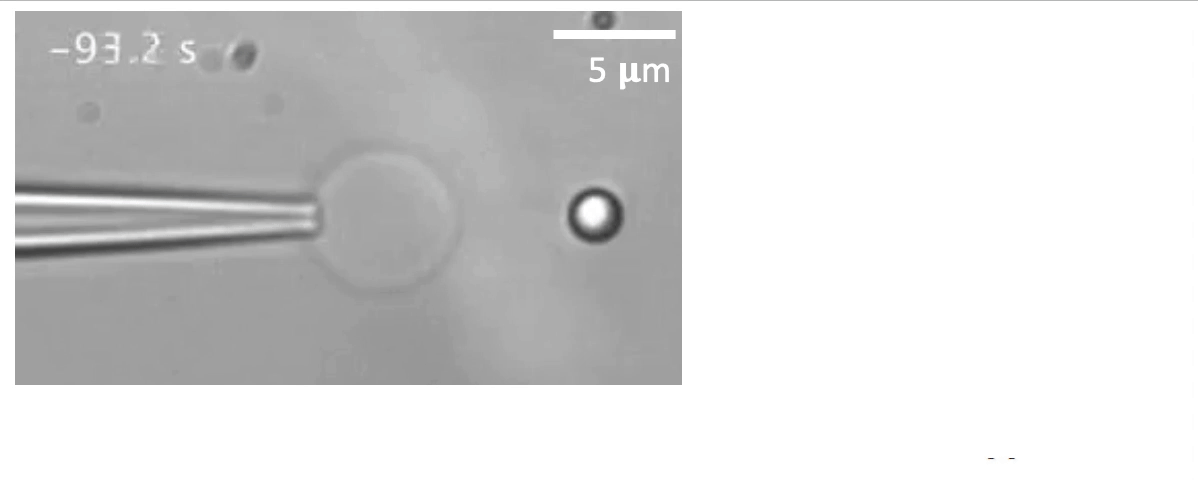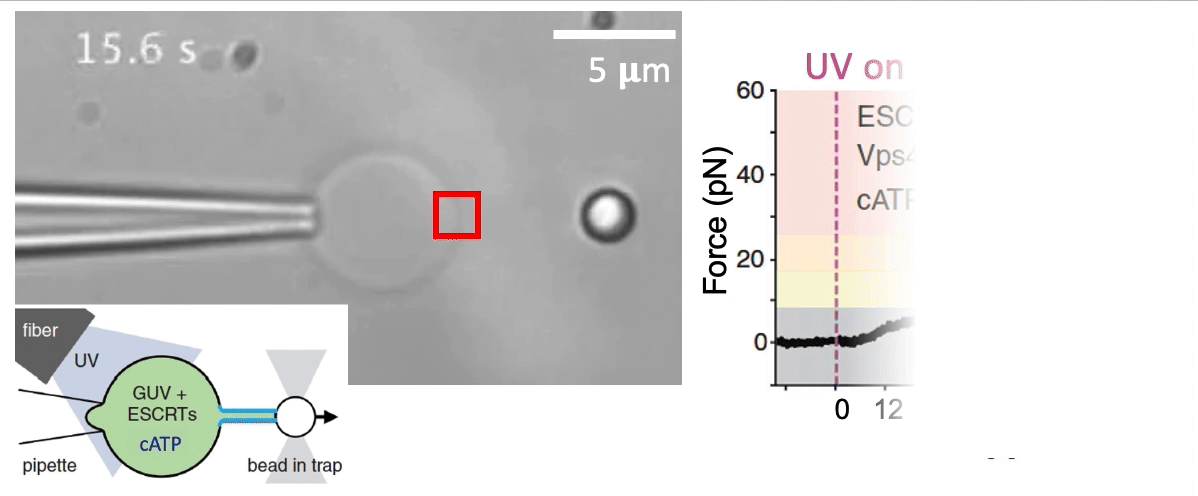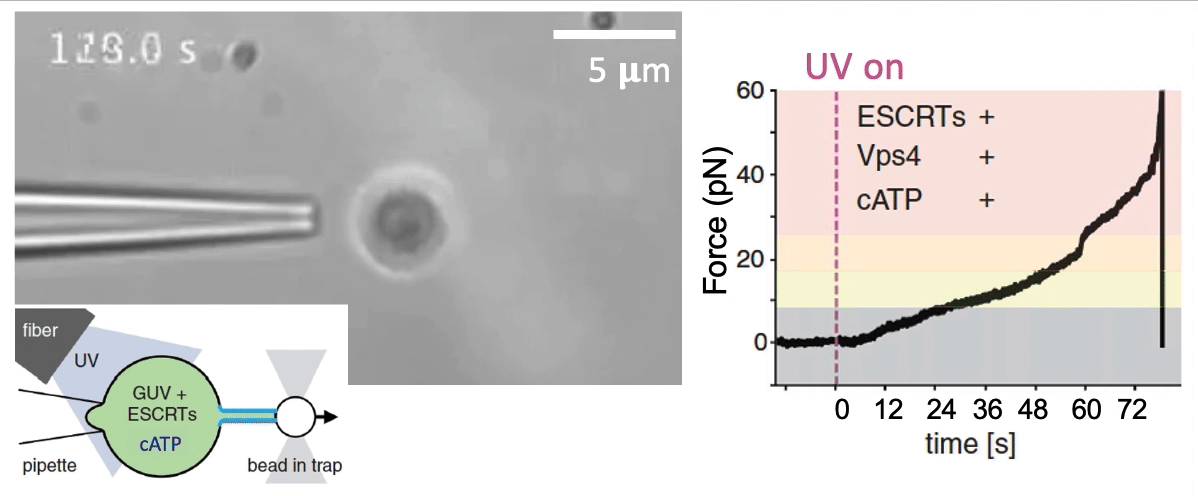
Recently, we succeed to study live cells force dynamics using adaptive high-resolution optical tweezers integrated with confocal fluorescence imaging module. In collaboration with the Weiner Lab at UCSF, we implemented optogenetic controls to modulate cellular activities on our force-probing/physical-manipulating platform (optical trapping plus micropipette aspiration; see videos above), which allows us to mechanically perturb the cells and simultaneously measure cellular forces such as change in membrane tension. We discovered that actin-driven protrusions/contractions in live human neutrophils generate rapid long-range membrane tension propagation across the cell, a property essential to the establishment of cell polarity during migration, and settled a long dispute in the field. This work also reveals that our membrane tether assay serves as an incisive approach to non-invasively resolve cortical tensions inside the cell, which becomes the rigorous basis to launch our studies on cortical dynamics during mitosis. By customizing our microfluidic setup, we expand the variety of cell types amenable to our system, ranging from mouse embryonic stem cells (mESCs) to an actively dividing sea star embryo (collaboration with the Barone Lab at Stanford).
How does the cell ‘physically’ divide? Detailed mechanical crosstalk between the membrane, cortex, and microtubule network allows the cell to coordinate temporarily and spatially its precise changes in morphology during mitosis and promote accurate segregation of genomic material. I aim to resolve the orchestration of cortical tension between the bipolar ends and the equator in symmetric cell division, interrogate how cortical tension dynamics impact spindle positioning and determine the division plane for cytokinetic furrow ingression, and ask whether a tension/force imbalance along the spindle may lead to chromosome misalignment. — Carly
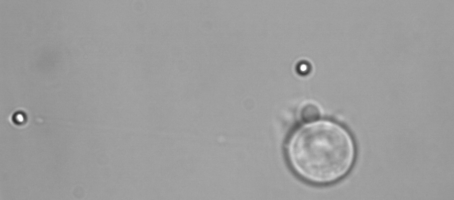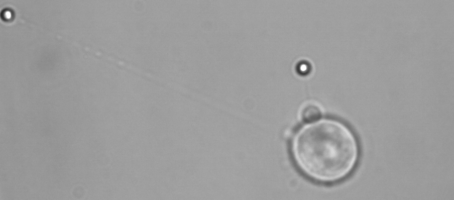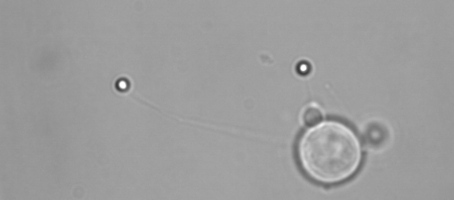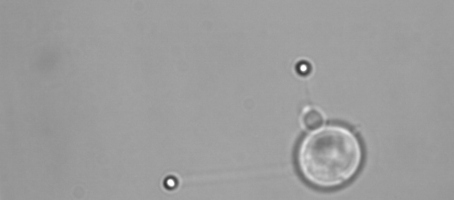
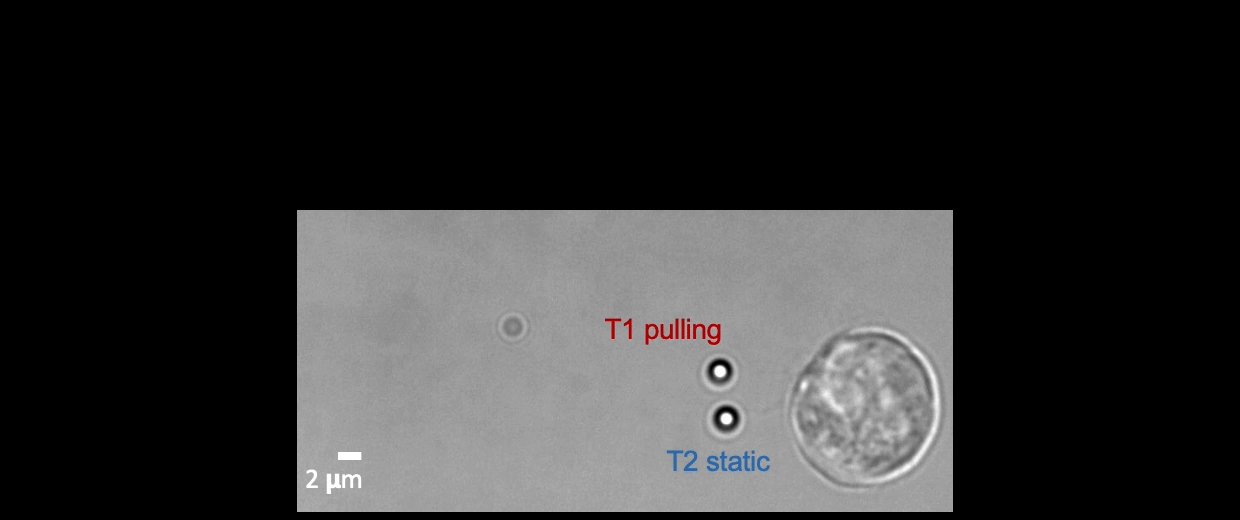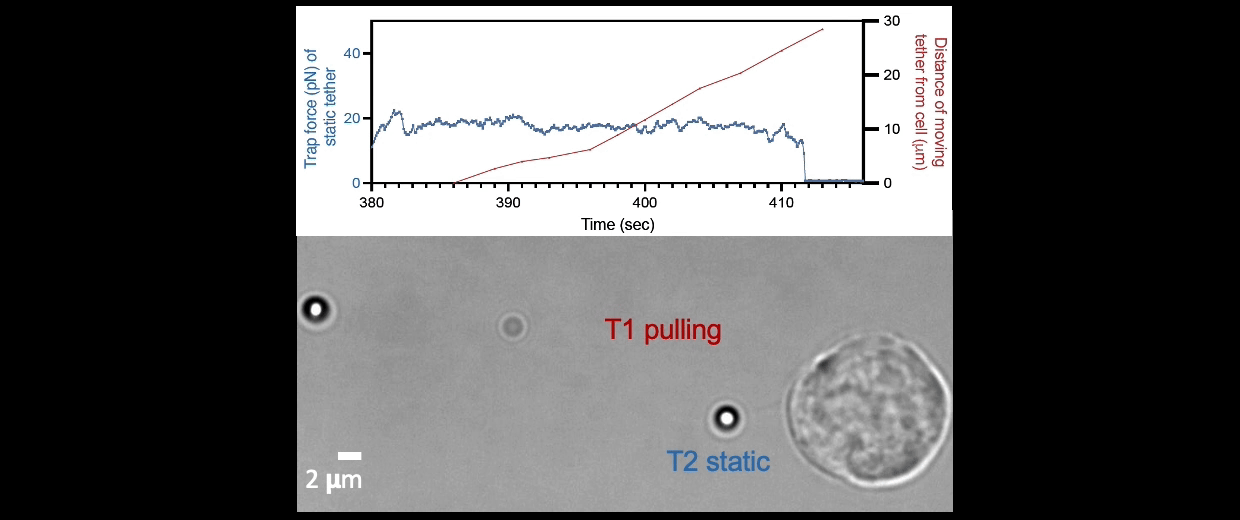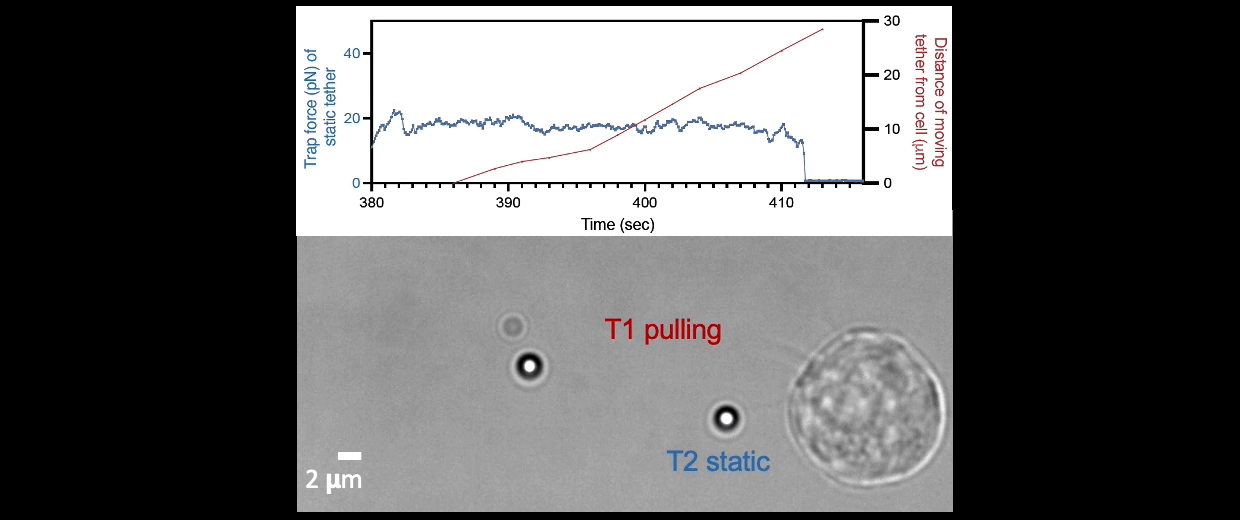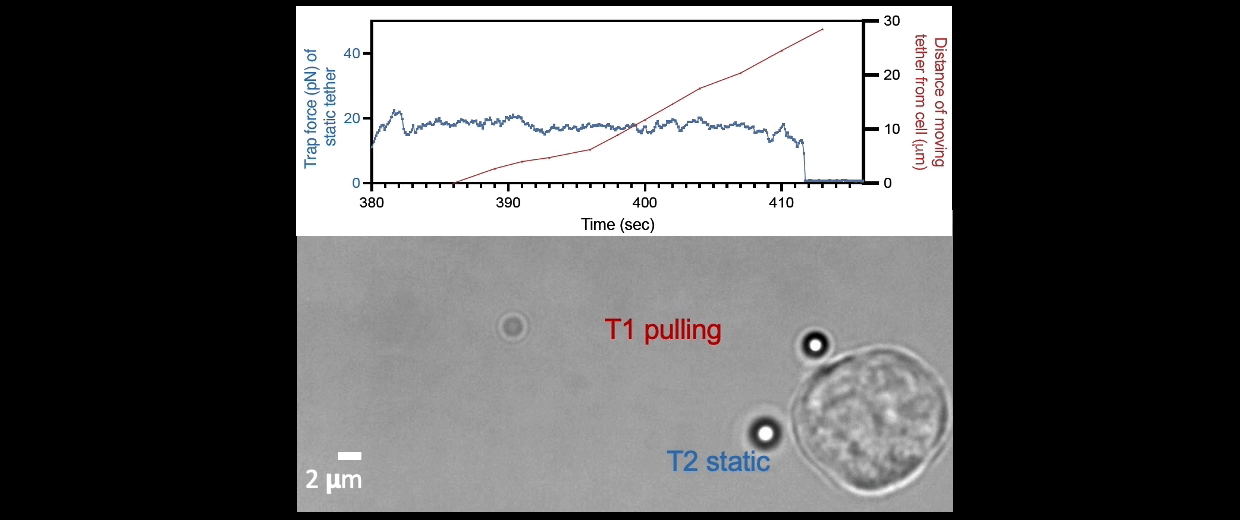
Membrane tether assays
to probe cortical tension
dynamics at multiple
locations around mESCs
How does the mitotic checkpoint proteins, MAD2, couple their metamorphic fold-switching dynamics to the precise orchestration of synchronized events during cell division? Its structural dynamics not only safeguards chromosome segregation, but also exceeds the predicting power of Alpha-Fold, and challenges the existing notion of ‘one-sequence-one-fold.’ In this project, we use single-molecule optical tweezers assay to resolve the dynamic folding trajectories for MAD2 transitions between its two functional states, which structural insight will guide us to unravel in live cells how MAD2 fold-switching flux oscillates—in time and space—accordingly with phases throughout mitosis. — Chuofan
Follow MAD2 fold switching
between open and closed forms
via optical tweezers assay
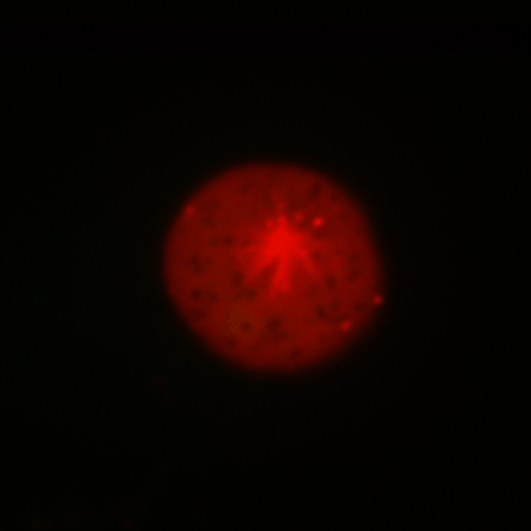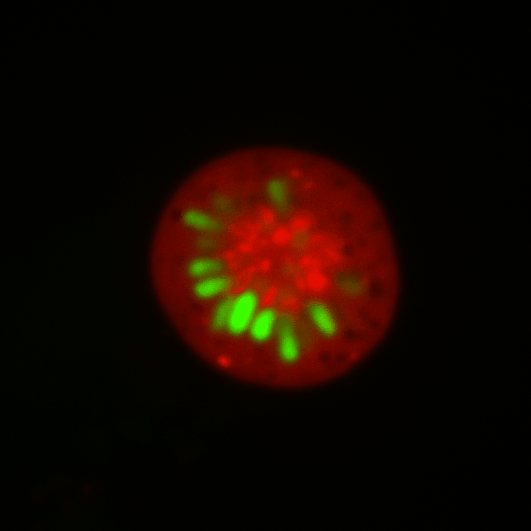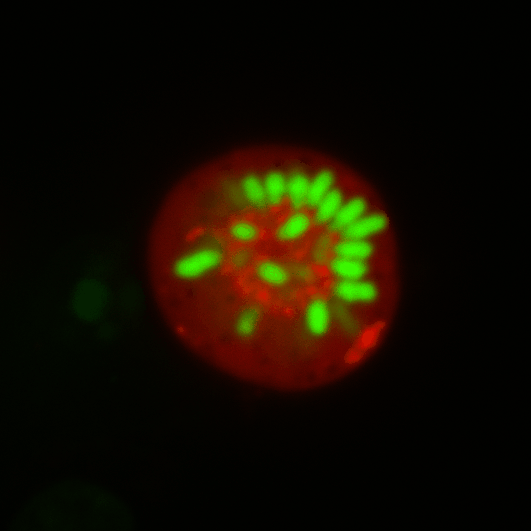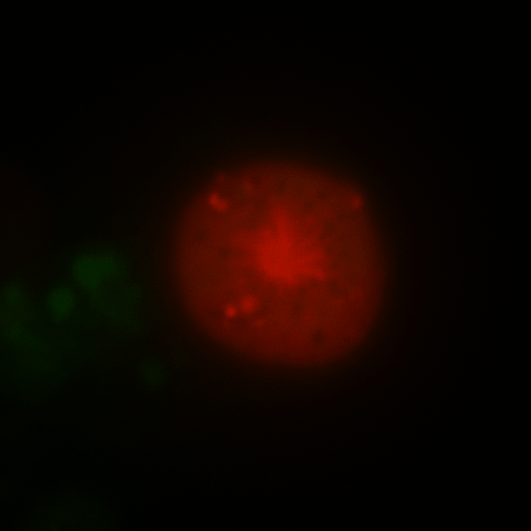
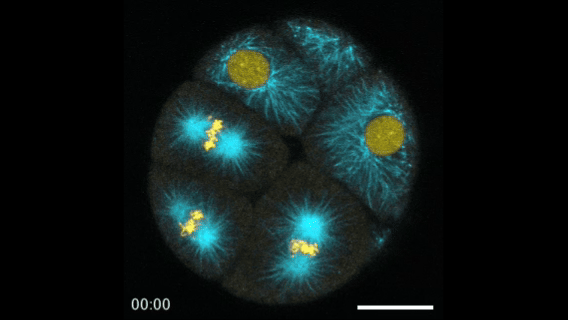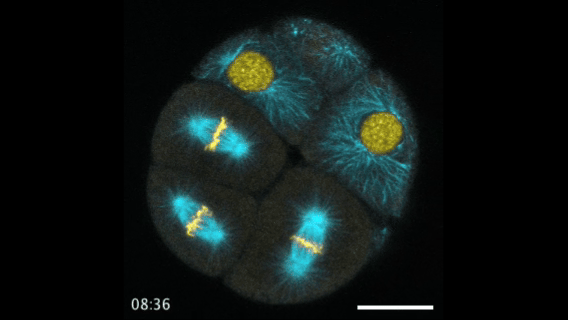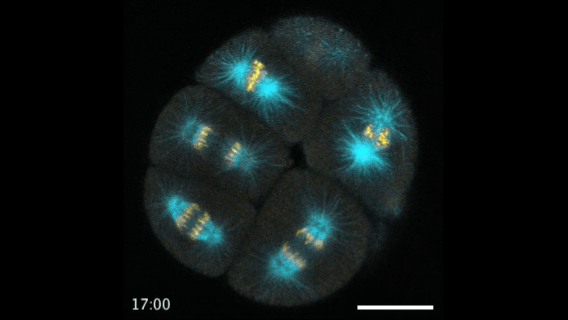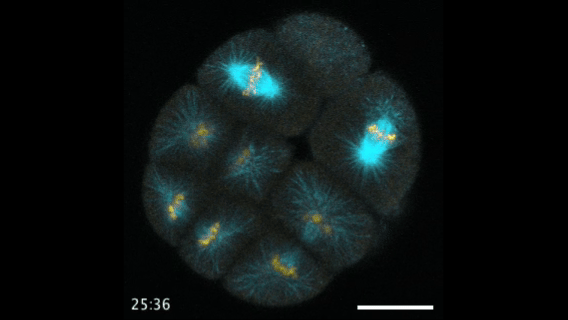
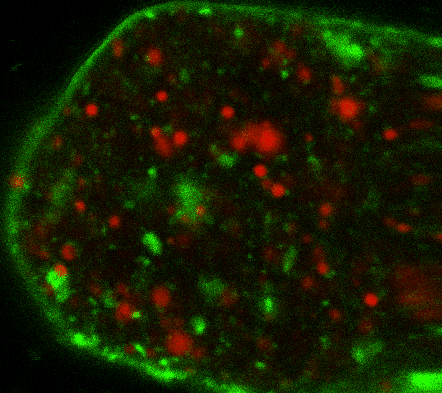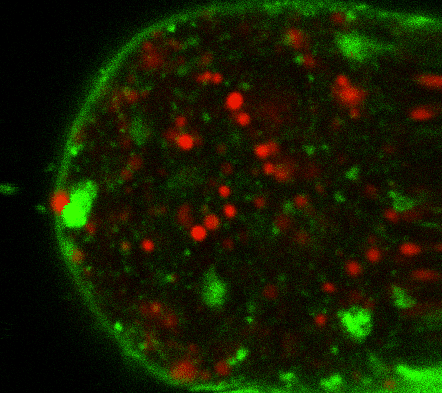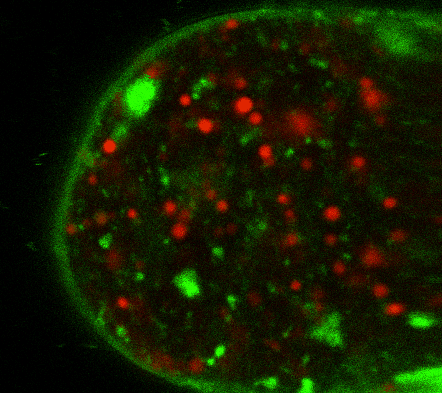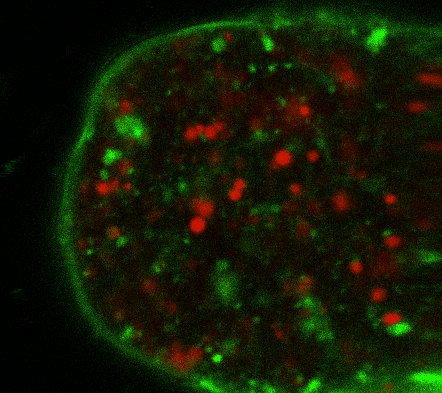
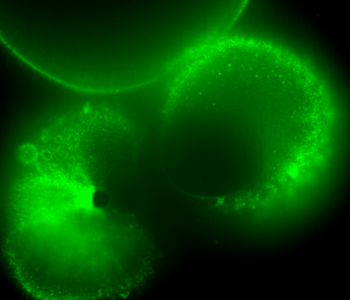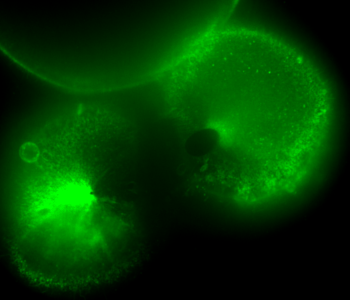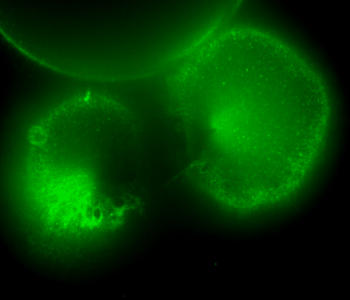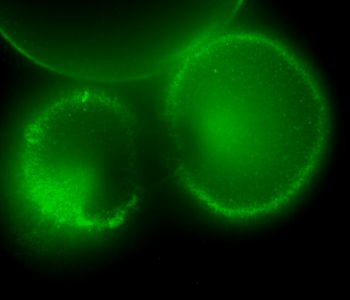
During early stage development, mitosis appears nearly concerted among cells within an embryo. How do the cortical tension dynamics evolve and coordinate between neighboring cells? Via live cell membrane tether assay in a dual trap experiment, we aim to simultaneously monitor the membrane tension profiles of cell pairs around a sea star embryo, thereby resolving their correlation and mechanical attribution to synchronicity in cell cycle. — June (joint with Barone Lab)
Synchronized furrow ingression between two neighboring mitotic cells (confocal z-slice recored at
the equator plane in real-
time; 2-to-4 cell stage).
As the mitotic cell elongates (top one; 4-to-8 cell stage), membrane tension increases near the polar end.
Ultimately, we want to study cellular mechanics in situ. Hence, we are also developing new genetically encoded force sensors and tension gauges, and constructing novel imaging platform ad hoc to directly visualize these hidden physical parameters present inside and exerted by the cells. For instance, the amount of forces transduced across each spindle fiber during mitosis, or a global map of membrane tension distribution as the entire embryo divides.
We will use optical tweezers to calibrate each molecular probe and identify constructs with proper dynamic ranges for probing cellular forces and tensions. The precise magnitude and dynamic coordination of these physical parameters are key to discern how cells interpret and integrate mechanical cues both from within and among interfacing neighbors, which is the core of our lab’s research. — Alice
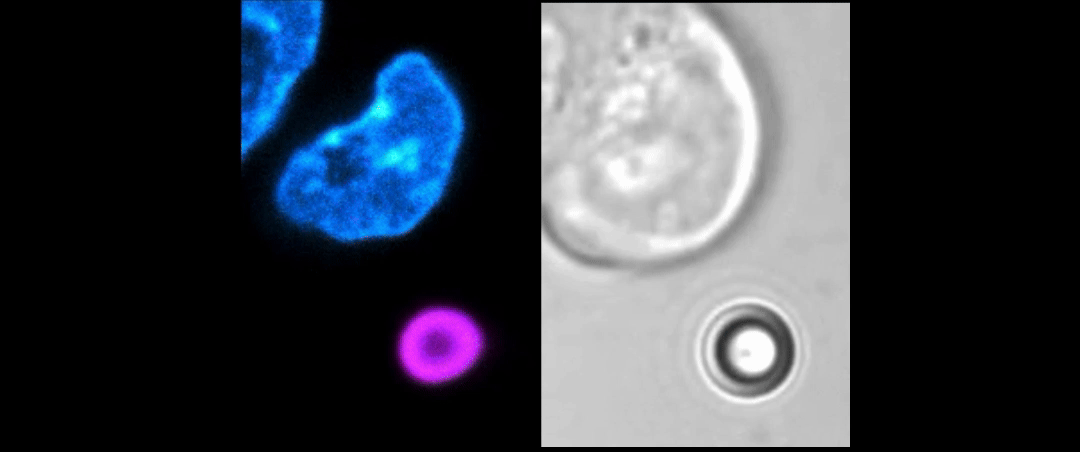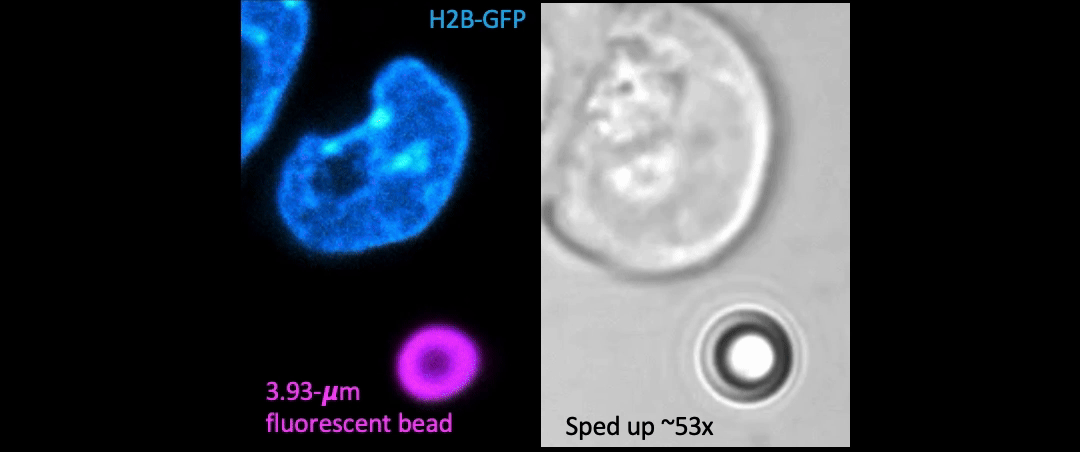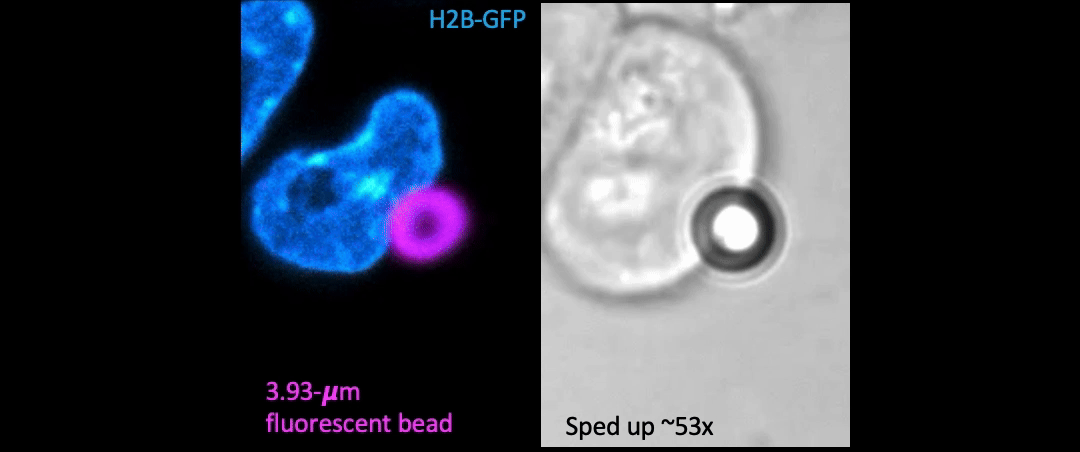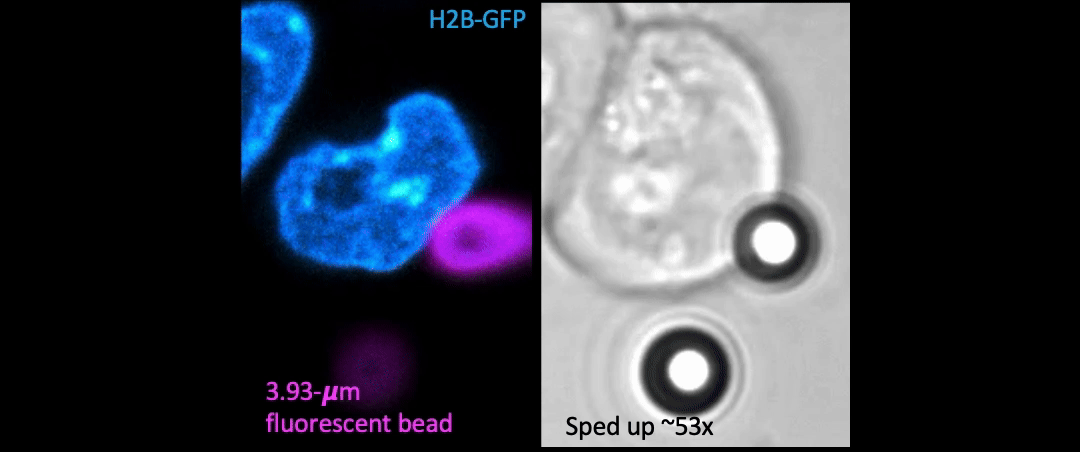
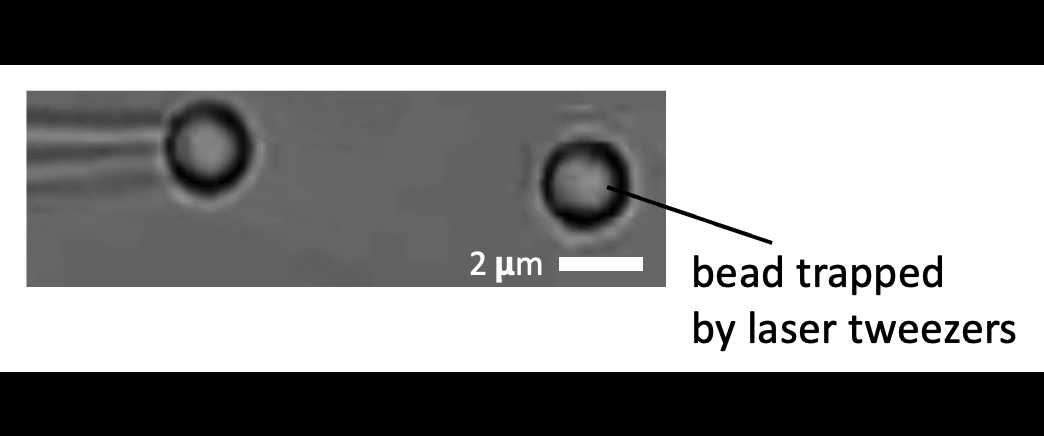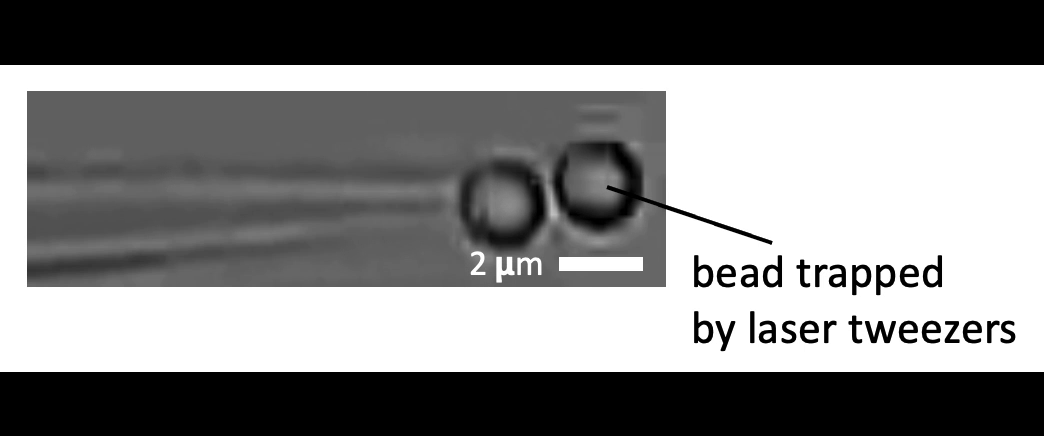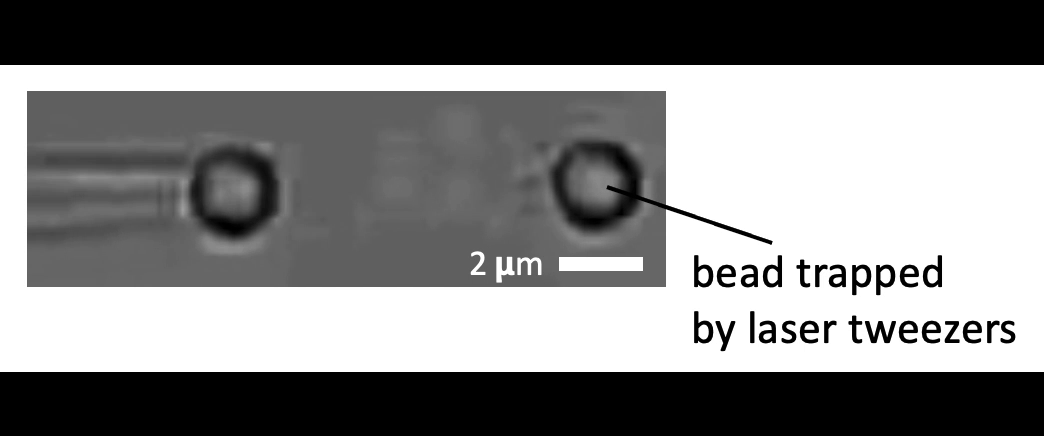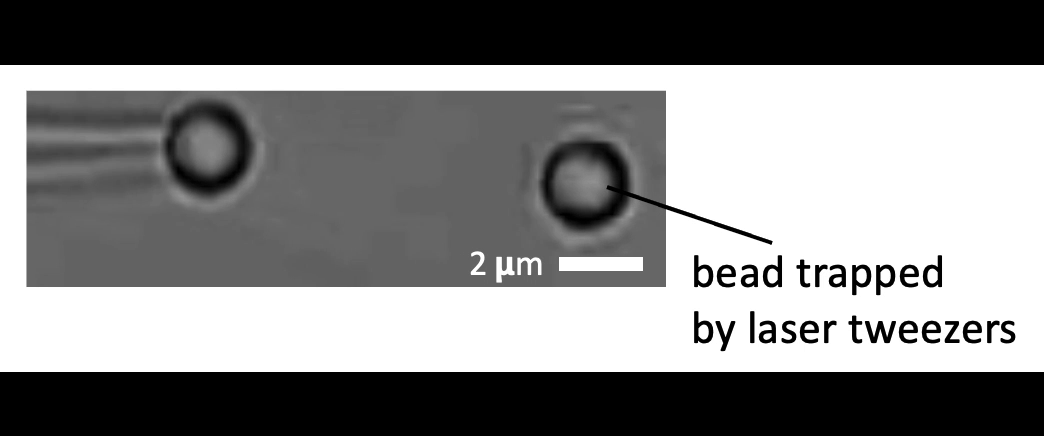
Given the presence of physical linkages (e.g., LINC complexes) between the cell membrane, cortex, and nucleus, could external physical stimuli—such as the intercellular mechanical crosstalk between neighboring cells within a developing embryo—have an intracellular impact by altering chromatin organization, gene accessibility, transcription dynamics, thereby giving cues for cell fate/differentiation? With the refined manipulating power of our adaptive optical tweezers instrument, we can apply physiological level of forces to indent or stretch nuclei in live mESCs and simultaneously monitor with confocal fluorescence imaging to study how mechanical perturbations impact chromatin organization, gene accessibility/expression, and transcription dynamics.
Mechanically indent and stretch the nuclei inside live cells with a micron-sized bead controlled by the laser trap. The extent of nucleus shape change produced by these external forces is similar to that when being compressed by a neighboring mitotic cell that elongates and expands in size (see left set of images).